1. New Ferroic Materials for Sensing, Energy Storage, and Energy Production
Ferroelectrics are insulating polar materials that demonstrate a macroscopic switchable polarization in an applied electric field. Many ferroelectrics are solid oxides based on the perovskite mineral structure whose properties have been engineering through compositional tuning. Ferroelectrics are crucial components in multi-billion dollar industries; they are can be found in non-volatile information storage technologies, SONAR, and communication devices. Their favorable mechanical responses have also led to their use in advanced sensing technology where small molecule adsorptions create a measurable change. We can also identify a pressing need to advance semiconductive ferroelectrics as photocatalysts for cleaner energy production; the polar ground state boosts the charge separation caused by solar illumination, and these materials have demonstrated some of the highest solar conversion efficiencies in the solid state.  The most urgent problem to solve in this field is to obtain synthesizable materials that operate in different regimes of temperature and pressure than current candidates, are inexpensive to synthesize, non-toxic, stable in air, and do not depolarize in thin film form. This means that searching for new materials, or known (often overlooked) mineral structures with new types of ferroelectric mechanisms, will be a highly advantageous starting point. Antiferroelectrics are related to ferroelectrics; they exhibit antipolar structural distortions that result in a net polarization of zero but can also be switched to a ferroelectric state. They find use as high-energy storage capacitors and high-strain actuators and transducers. We will explore the emerging field of antiferroelectrics as materials capable of improved energy storage. Antiferroelectrics have a highly nonlinear change in volume and entropy associated with the phase transition between antipolar and polar states. This means that we can store and release energy by making and breaking chemical bonds!
The most urgent problem to solve in this field is to obtain synthesizable materials that operate in different regimes of temperature and pressure than current candidates, are inexpensive to synthesize, non-toxic, stable in air, and do not depolarize in thin film form. This means that searching for new materials, or known (often overlooked) mineral structures with new types of ferroelectric mechanisms, will be a highly advantageous starting point. Antiferroelectrics are related to ferroelectrics; they exhibit antipolar structural distortions that result in a net polarization of zero but can also be switched to a ferroelectric state. They find use as high-energy storage capacitors and high-strain actuators and transducers. We will explore the emerging field of antiferroelectrics as materials capable of improved energy storage. Antiferroelectrics have a highly nonlinear change in volume and entropy associated with the phase transition between antipolar and polar states. This means that we can store and release energy by making and breaking chemical bonds!
Finding these missing materials would be the key to unlocking access to new frontiers in science, engineering, and industry. Here are a few of our recent studies in the field of ferroic predictions:
Data-Enabled Discovery of Two-Dimensional van der Waals Layered Phosphochalcogenides; Peng Yan, Mona Layegh, Ryan Stadel, Joshua Birenzvige, Peter Y. Zavalij, Efrain E. Rodriguez, and Joseph W. Bennett, Chem. Mater., 2025, 37, 14, 5086–5098 (link)
Assessing the K2BO3 Family of Materials as Multiferroics; Anthony A. Casale and Joseph W. Bennett, Physical Review Materials, 2024 (8) 114424 (link)
Developing New Antiferroelectric and Ferroelectric Oxides and Chalcogenides Within the A2BX3 Family; A. C. Khan, A. S. Cook, J. A. Leginze, and J. W. Bennett*, J. Mater. Res., 2022, (37), 346-359 (link) *Special Issue Highlighting Early Career Materials Scientists 2022
2. New Classes of Chemically Resistant Materials for Batteries, Water Treatment, and Potential Delivery Systems
Industrial funding towards scalable aqueous-based battery chemistries for grid-scale energy storage has increased as both the safety and scalability of solid state Li-ion batteries has been called into question. However, current electrodes for aqueous batteries are at most times expensive (Ir/Ru/Ti-based) and prone to degradation over the course of their lifetime. 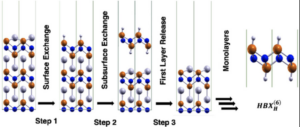 Most aqueous electrode corrosion happens as the pH changes over the course of charge/discharge cycles, and this is exacerbated if the changes in pH produce highly oxidizing species. We would prefer many inexpensive, durable, environmentally benign alternatives that are stable to a variety of operating conditions, as material feedstock costs may fluctuate rapidly.
Most aqueous electrode corrosion happens as the pH changes over the course of charge/discharge cycles, and this is exacerbated if the changes in pH produce highly oxidizing species. We would prefer many inexpensive, durable, environmentally benign alternatives that are stable to a variety of operating conditions, as material feedstock costs may fluctuate rapidly.
A critical first step in uncovering these materials is to calculate the surface transformation thermodynamics of a wide range of materials that include mineral oxides, sulfides, and pnictides. Here are a few of our recent studies on surface phenomena:
The Formation and Stability of 3D and 2D Materials; Mona Layegh, Peng Yan, Joseph W. Bennett, Progress in Crystal Growth and Characterization of Materials, 2024 (70), 100615 (link)
The Effects of Chlorine-Containing Species on Cinnabar: A Density Functional Theory Investigation into the Surface Adsorption Reactivity of Mercury Sulfide; Aria Tauraso, G. Amalthea Trobare, Lillian G. Kidd, Jessica E. Heimann, Zeev Rosenzweig, Joseph W. Bennett*, Surface Science, 2024 (740), 122412 (link) *Featured in the Young Investigator Special Issue 2023
Chemical Transformations of 2D Kaolinic Clay Mineral Surfaces from Sulfuric Acid Exposure; Chari, Celia; Heimann, Jessica; Rosenzweig, Zeev; Bennett, Joseph W.*; Faber, Katherine, Langmuir, 2023 (39), 6964-6974 (link)
A DFT Combined with Thermodynamics Exploration of Novel 2D Materials Created Using Aqueous Exfoliation; Mona Layegh and Joseph W. Bennett*,J. Phys. Chem. C., 2023 (127) 2314-2325 (link) *Special issue “Early Career and Emerging Researchers in Physical Chemistry”
3. New Absorbent Platforms for Water Treatment and First-Principles Insights Into Public Water Systems
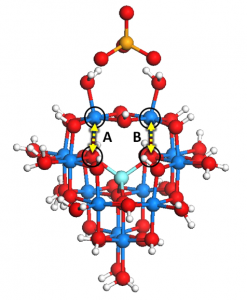 Water contamination is a devastating human health problem. Three of the most common inorganic groundwater contaminants are arsenate, phosphate, and nitrate. The amount of research devoted to arsenic and phosphate remediation far exceeds the amount of literature devoted to nitrate removal, even though its effects on human health and the environment are just as devastating. To solve this problem, two options are either nitrate-selective adsorbent surfaces or nitrate selective sequestration. The former option may prove difficult, since nitrate is classified as a weak monodentate outer-sphere ligand and can easily be displaced by bidentate inner sphere ligands that tend to form stronger bonds to an oxide surface, such as arsenate. My recent work on anion adsorption on naturally occurring aluminum nanoclusters shows that the difference between nitrate and phosphate adsorption can be anywhere between 2.5 to 4.5 eV, favoring phosphate in all cases. If we consider the fact that nitrate has a smaller ionic radius (179 pm) than either phosphate (238 pm) or arsenate (248 pm), then nitrate selective sequestration may be a more tractable solution than competitive adsorption.
Water contamination is a devastating human health problem. Three of the most common inorganic groundwater contaminants are arsenate, phosphate, and nitrate. The amount of research devoted to arsenic and phosphate remediation far exceeds the amount of literature devoted to nitrate removal, even though its effects on human health and the environment are just as devastating. To solve this problem, two options are either nitrate-selective adsorbent surfaces or nitrate selective sequestration. The former option may prove difficult, since nitrate is classified as a weak monodentate outer-sphere ligand and can easily be displaced by bidentate inner sphere ligands that tend to form stronger bonds to an oxide surface, such as arsenate. My recent work on anion adsorption on naturally occurring aluminum nanoclusters shows that the difference between nitrate and phosphate adsorption can be anywhere between 2.5 to 4.5 eV, favoring phosphate in all cases. If we consider the fact that nitrate has a smaller ionic radius (179 pm) than either phosphate (238 pm) or arsenate (248 pm), then nitrate selective sequestration may be a more tractable solution than competitive adsorption.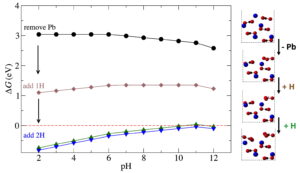
For optimal nitrate sequestration, we require either a porous tunnel structure with adjustable pore dimensions, or a layered structure with tunable interplanar distances that would selectively accommodate a nitrate anion. We also require tunable electrostatic interactions, as negatively charged nitrate would preferentially bind to a positively charged surface, or a structure in which electron density can be delocalized. Ideal adsorption should be moderately strong (up to 1-1.5 eV), but weak enough that we can remove the nitrate to be able to reuse the adsorbent sequestration material in an effort towards designing sustainable technologies. Beyond designing materials for nitrate removal, this research thrust could be extended to other polyatomic anions with multiple speciation states such as SeO42- and PO43- or cations such as Pb2+ and Cu2+. These ions have been the subject of recent media coverage, especially the massive public health concerns about the quality of drinking water. Coupled closely to this is the need to better understand how similar planar anions, like carbonate, behave when in contact with water. Here are a few of our recent studies on carbonate surfaces in water:
Surface Transformation Thermodynamics of Alkaline Earth Carbonates using First-Principles Calculations; Ryan T. Grimes and Joseph W. Bennett*, Surface Science, 2022 (726) 122165 (link) *Selected for the cover of the December 2022 issue of Surface Science
Surface Transformations of Lead Oxides and Carbonates Using First-Principles and Thermodynamics Calculations; R. T. Grimes, J. A. Leginze, R. Zochowski, and J. W. Bennett*,Inorg. Chem., 2021 (60) 1228-1240 (link) *Featured in the “Out in Inorganic Chemistry: A Celebration of LGBTQIAPN+ Inorganic Chemists” Issue of Inorganic Chemistry: Out in Inorganic Chemistry Special Issue